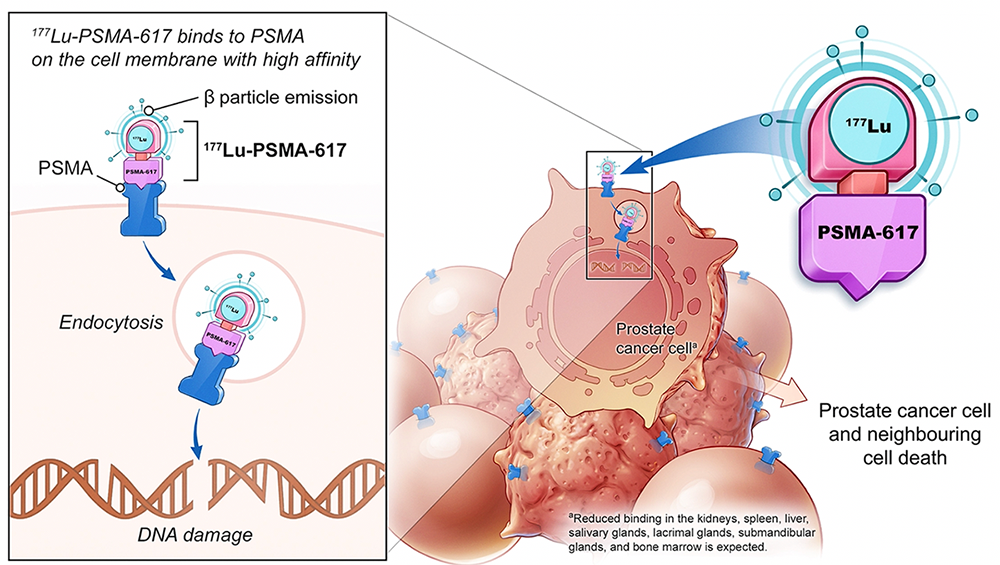
ASCO 2021: Phase III Study of Lutetium-177-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer (VISION)
This is the newest, state-of-the-art treatment for metastatic prostate cancer. It’s called “theranostics” which targets prostate cancer cells using prostate-specific membrane antigen (PSMA)-targeting radioligands. PSMA is highly expressed in prostate cancer, the combination of PSMA-617 with the beta-emitter lutetium allows for the targeted delivery of ß-particle radiation to PSMA-expressing cells and the surrounding microenvironment. One of the most important prostate cancer clinical trials to be published in the last decade is the VISION trial, a phase III study assessing lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (mCRPC).
The VISION trial is an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy (NCT03511664). Additionally, patients must have had an ECOG performance status of 0-2 and life expectancy of at least 6 months.
Additional inclusion criteria:
—Patients must have had PSMA-positive metastatic disease on the basis of a PSMA PET scans
—No PSMA-negative metastatic lesions
Results:
—Treatment with 177Lu-PSMA-617 + standard of care (SOC) treatments significantly improved overall survival (OS) by a median of 4.0 months (median overall survival, 15.3 vs 11.3 months compared to SOC alone.
—Radiographic progression free survival (rPFS) was significantly improved by a median 5.2 months (8.8 vs 3.6 months compared to SOC alone.
Conclusions:
—The VISION trial demonstrates that 177Lu-PSMA-617 can be safely added to SOC treatment and improves rPFS and prolongs OS compared with SOC alone in men with advanced-stage PSMA-positive mCRPC, supporting its adoption as a standard of care.
—The results of this trial impacted practice globally. Even without formal US FDA approval (anticipated in the next few months), this therapy has been available in other countries, and we’ve seen patients who really want access to this drug leaving the U.S. and going to those countries to be treated.
https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-prostate-cancer/130016-asco-2021-vision.html?fbclid=IwAR2A0asjlI0tdtysrz-DjaE_wcg6aYth2K3D-B4B9fK0opQ3hVBYK2LgdTs